

Cold-water carbonates do exist at higher latitudes but have a very slow growth rate. Calcium carbonate contributors, including plankton (such as coccoliths and planktic foraminifera), coralline algae, sponges, brachiopods, echinoderms, bryozoa and mollusks, are typically found in shallow water environments where sunlight and filterable food are more abundant.
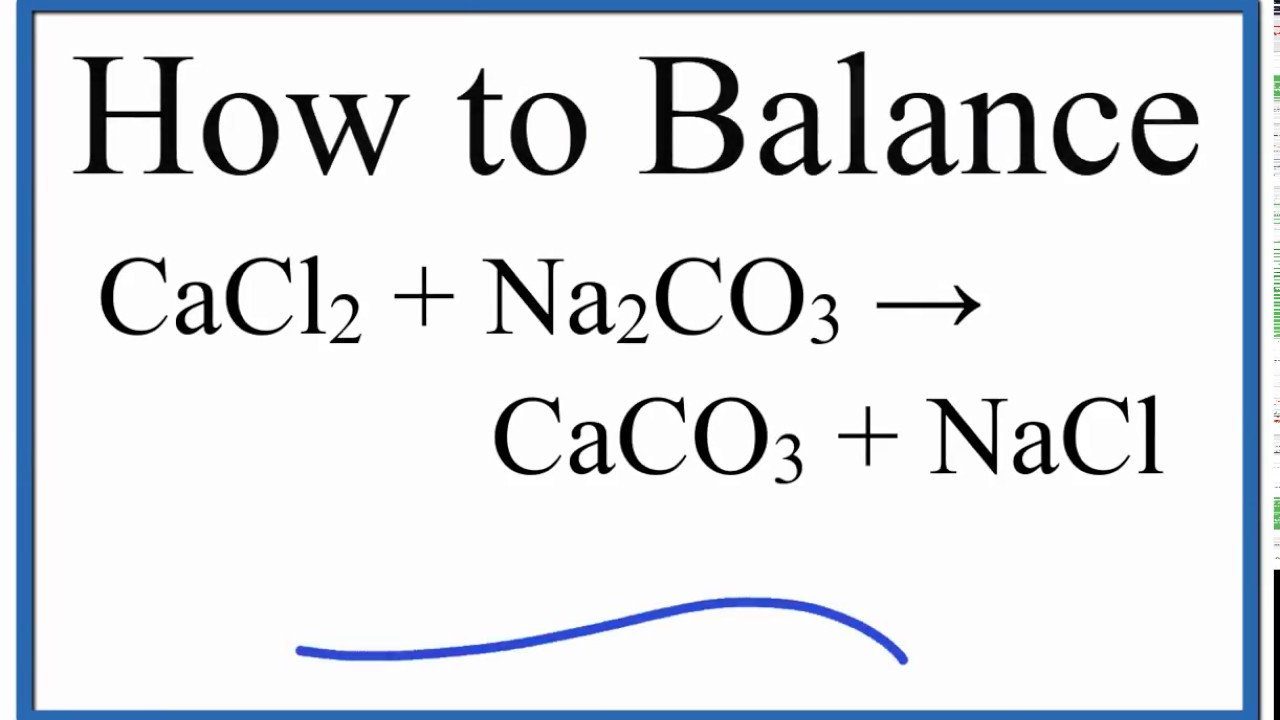
In warm, clear tropical waters corals are more abundant than towards the poles where the waters are cold. The carbonate minerals form the rock types: limestone, chalk, marble, travertine, tufa, and others. Calcium carbonate occurs as aragonite, calcite and dolomite as significant constituents of the calcium cycle. Geology Surface precipitation of CaCO 3 as tufa in Rubaksa, EthiopiaĬarbonate is found frequently in geologic settings and constitutes an enormous carbon reservoir. This provides some evidence for the past presence of liquid water. Signs of calcium carbonate have been detected at more than one location (notably at Gusev and Huygens craters). Extraterrestrial īeyond Earth, strong evidence suggests the presence of calcium carbonate on Mars. Dark green vegetables such as broccoli and kale contain dietarily significant amounts of calcium carbonate, but they are not practical as an industrial source. Oyster shells have enjoyed recent recognition as a source of dietary calcium, but are also a practical industrial source. Industrially important source rocks which are predominantly calcium carbonate include limestone, chalk, marble and travertine.īiological sources Calcium carbonate chunks from clamshellĮggshells, snail shells and most seashells are predominantly calcium carbonate and can be used as industrial sources of that chemical. Geological sources Ĭalcite, aragonite and vaterite are pure calcium carbonate minerals. A transparent variety called Iceland spar (shown here) was used to create polarized light in the 19th century. Occurrence Calcite is the most stable polymorph of calcium carbonate. The ability of phase selection is usually attributed to the use of specific macromolecules or combinations of macromolecules by such organisms. Moreover, they exhibit a remarkable capability of phase selection over calcite and aragonite, and some organisms can switch between the two polymorphs. Organisms, such as molluscs and arthropods, have shown the ability to grow all three crystal polymorphs of calcium carbonate, mainly as protection (shells) and muscle attachments. Some polyamines such as cadaverine and Poly(ethylene imine) have been shown to facilitate the formation of aragonite over calcite. For example, the formation of aragonite is promoted by the presence of magnesium ions, or by using proteins and peptides derived from biological calcium carbonate. Microscopic Calcite and VateriteĪragonite occurs in majority when the reaction conditions inhibit the formation of calcite and/or promote the nucleation of aragonite. However, aragonite, whose stability lies between those of vaterite and calcite, seems to be the exception to this rule, as aragonite does not form as a precursor to calcite under ambient conditions. This behavior seems to follow Ostwald's rule, in which the least stable polymorph crystallizes first, followed by the crystallization of different polymorphs via a sequence of increasingly stable phases. In additive-free aqueous solutions, calcite forms easily as the major product, while aragonite appears only as a minor product.Īt high saturation, vaterite is typically the first phase precipitated, which is followed by a transformation of the vaterite to calcite. Crystallization Crystal Structure of Calcite and AragoniteĪll three polymorphs crystallize simultaneously from aqueous solutions under ambient conditions. reacts with acids, releasing carbon dioxide (technically speaking, carbonic acid, but that disintegrates quickly to CO 2 and H 2O):ĬaCO 3 ( s ) + 2 H + ( aq ) ⟶ Ca 2 + ( aq ) + CO 2 ( g ) + H 2 O ( l ), the minor structure is still unknown.Chemistry Ĭalcium carbonate shares the typical properties of other carbonates. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues. Calcium carbonate is the active ingredient in agricultural lime and is produced when calcium ions in hard water react with carbonate ions to form limescale. Materials containing much calcium carbonate or resembling it are described as calcareous. It is a common substance found in rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skeletons and pearls. Chemical compound Crystal structure of calciteĬalcium carbonate is a chemical compound with the chemical formula Ca CO 3.


 0 kommentar(er)
0 kommentar(er)
